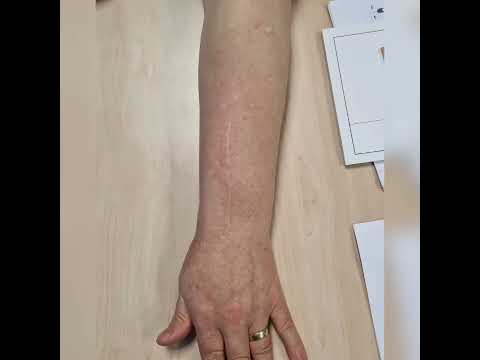
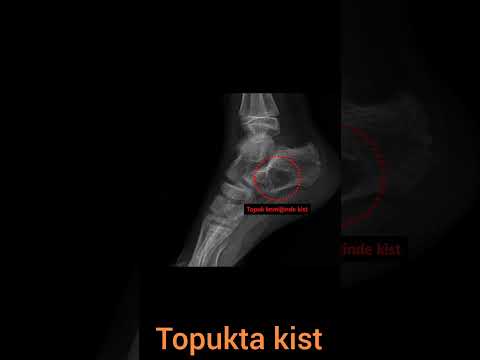
Use of cement combined grafting in upper and lower extremity benign bone tumors
Anıl Pulatkan, MD , Vahdet Uçan, MD , Sevil Tokdemir,
MD , Nurzat Elmalı, MD , Volkan Gürkan, MD
Department of Orthopedics and
Traumatology, Bezmialem Vakıf University School of Medicine, Istanbul, Turkey
Department of Radiology, Bezmialem
Vakıf University School of Medicine, Istanbul, Turkey
There
is no standard procedure for the treatment of benign bone tumors. The bone
defect following the curettage of the bone tumor can be filled with autologous
bone marrow, polymethylmethacrylate cement, allograft, tricalcium phosphate,
and demineralized bone matrix (DBM). All these procedures have their own
advantages and disadvantages. Autografting is the gold standard in tumor
surgery; nevertheless, its disadvantages including limited access, cosmetic
problems, and donor site morbidity make the alternative treatment modalities as
viable options. Resorption of graft material and transmission of disease are
associated risks of allograft us Polymethylmethacrylate cement is
non-biological and its Young’s modulus of elasticity is lower than cortical
bone, responds to compression-distraction forces differently compared with
cortical bone, and has poor tensile and shear strength. Demineralized bone
matrix is expensive and osteoinductive without structural support.
Our
hypothesis was that cement combined DBM treatment stimulates new bone
formation, thus improves the functional scores. To the authors' knowledge, no
study has focused on this technique and searched the effect of new bone
formation in the cortical window on functional outcomes. Therefore, in this study,
we aimed to investigate the effectivity of cement combined DBM treatment on new
bone formation in the cortical window as well as to evaluate the effect of new
bone formation on functional outcomes.
PATIENTS AND METHODS
Thirty-two
benign bone tumor patients (15 males, 17 females; median age 38 years; range,
12 to 68 years), who underwent cement combined DBM procedure at Bezmialem Vakıf
University School of Medicine between February 2010 and December 2014 and were
followed up for a minimum of one year, were evaluated retrospectively. Patients
with axial (n=2), pelvic bone tumors (n=3), metastatic giant cell bone tumor
(n=2), or those who underwent adjuvant radiotherapy or chemotherapy (n=1) or
were followed up for less than one year (n=11) were excluded. The study
protocol was approved by the Bezmialem Vakıf University School of Medicine
Ethics Committee. A written informed consent was obtained from each patient.
The study was conducted in accordance with the principles of the Declaration of
Helsinki.
The
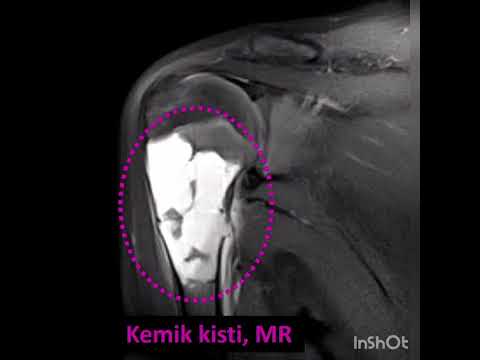
mean follow-up time was 20.8±7.7 months. There were simple bone cysts (n=6,
19%), enchondromas (n=14, 43%), aneurysmal bone cyst (n=1, 3%), fibrous
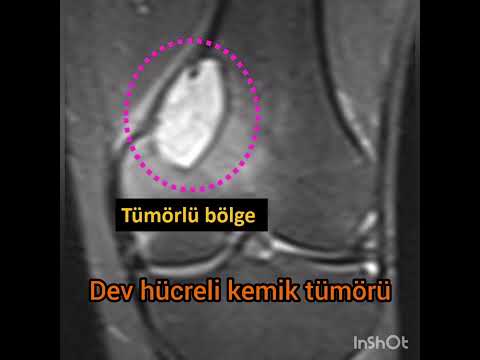
dysplasia (n=3, 9%), chondroblastomas (n=2, 6%), and giant cell bone tumors
(n=6, 19%) according to the pathology results.
The
lesions were located at the proximal humerus (n=5), proximal femur (n=3),
distal femur (n=16), proximal tibia (n=5), distal tibia (n=1), and calcaneus
(n=2). There were three (9%) Enneking stage I, 16 (50%) stage II, and 13 (41%)
stage III patients.
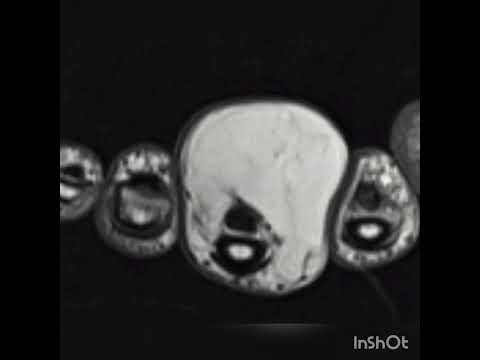
All
patients were examined through direct X-ray, computed tomography (CT), and
magnetic resonance imaging (MRI) for preoperative surgical planning. All
operations were performed by the same experienced tumor surgeon and the
operation procedure was similar. A tourniquet was used in all patients if tumor
localization allowed. Generally, an adequate longitudinal incision was
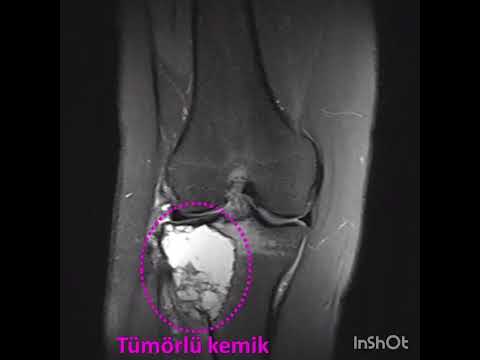
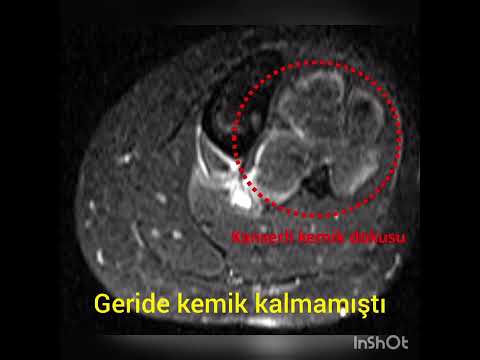
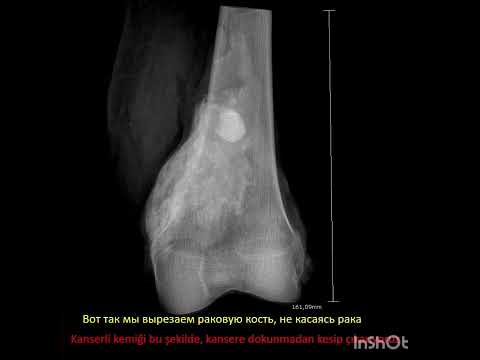
performed over the lesion to dominate the whole lesion. An oval cortical window
was created with a drill and osteotome. The cortical window and affected soft
tissue on the cortex were removed. After an extensive curettage was performed,
mechanical cleaning was carried out with a high[1]speed burr. If necessary,
the cavity was rinsed with phenol and ethanol solution while preserving the
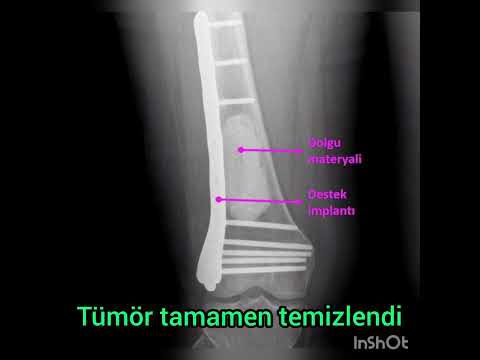
surrounding soft tissue. Then, antibiotic-free bone cement was prepared and the
cavity was filled with high viscosity bone cement (Biomet Bone Cement R, Biomet
Orthopedics GmbH, Ried, Switzerland). Grooves were created with a scalpel on
the surface of cement to increase the cement-graft retention. Thereafter, when
the cement was solidified, putty form of DBM (Grafton, Osteotech Inc.,
Eatontown, NJ, USA) was applied with at least one standard cortical thickness
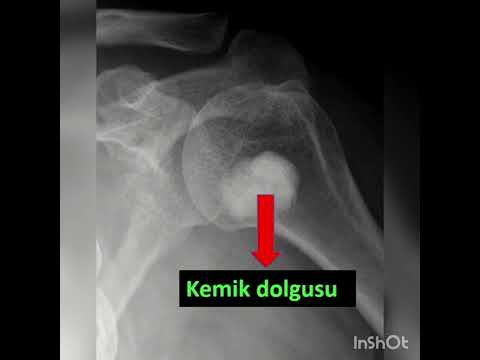
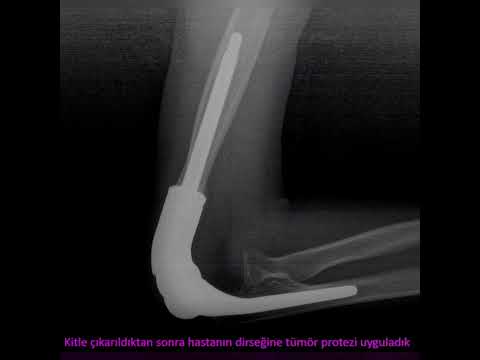
on the cement (Figure 1). Prophylactic osteosynthesis was performed in patients
with possible pathological fracture.
Was
calculated according to the direct preoperative X-rays and CT sections.[4]
Patients were routinely controlled with direct radiography every three months
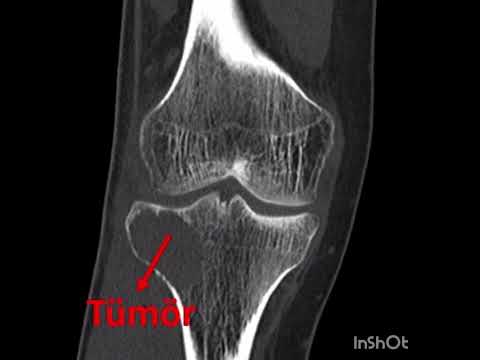
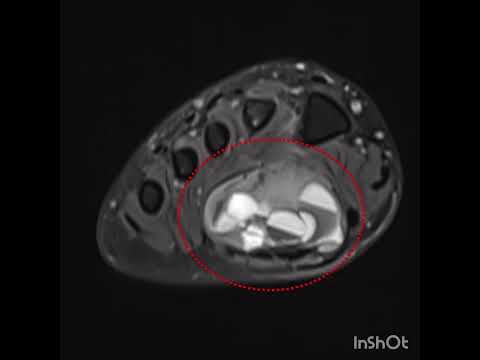
for the first year. To assess tumor recurrence and bone regeneration on the
cortical window, all patients were evaluated with CT scans in the first
postoperative year (Figure 2). As there is no defined classification method in
the literature, we used our own methodology to classify the amount of new bone
formation on the cortical window regarding CT scans (Table I). Every
measurement on radiological.
images
was performed by a radiologist three times to reduce the dating error.
Musculoskeletal Tumor Society (MSTS) functional scores of all patients were
performed in the first postoperative year.
The
relationship between new bone formation on the cortical window, age, Enneking
tumor stage, functional score, time to return work, size of the cortical window
(cm2 ), tumor size (cm3 ), and usage of prophylactic fixation were evaluated.
Statistical analysis
Statistical
analysis of the data was performed using the IBM SPSS version 21.0 software
(IBM Corp., Armonk, NY, USA). Concordance of the continuous data to normal
distribution was tested by Shapiro[1]Wilk
test. Continuous variables were expressed with median (minimum-maximum) and
mean ± standard deviation values and categorical variables were expressed with
frequency (percentage) values. Two group comparisons were performed using the
Mann-Whitney U test; independent sample t-test and three group comparisons were
performed using the Kruskal-Wallis and one-way analysis of variance tests. The
relationship between non-normally.
distributed
variables was investigated by Spearman's correlation coefficient. Results were
reported with 95% confidence intervals (CI) and related p values. P<0.05 was
considered as statistically significant.
RESULTS
The
median size of the cortical window to reach the tumor was 8.3 cm2 (range, 1.6
to 28.4 cm2 ), while the median tumor volume was 17.2 cm3 (range, 2.8 to 139.6
cm3 ). The median time to return to work was 60 days (range, 15 to 220 days).
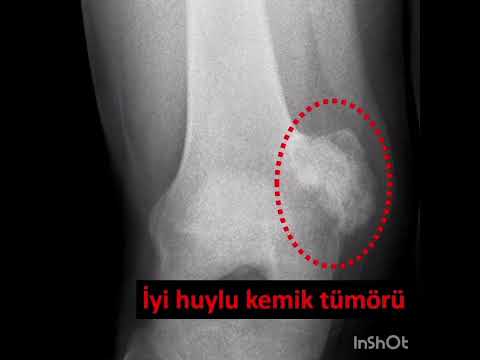
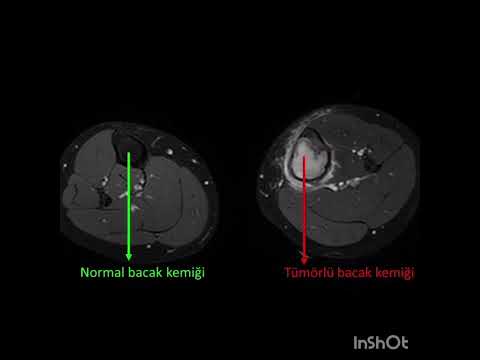
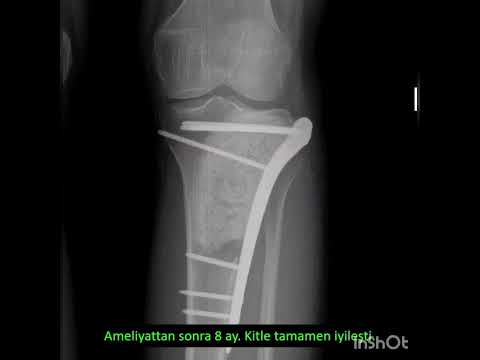
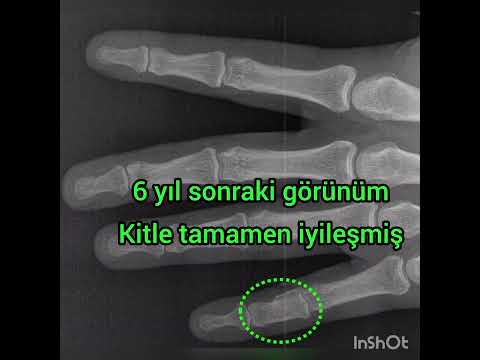
The median new bone formation on the cortical window was grade II. Ten
patients’ cortical windows were totally healed with the new bone formation
(grade IV) and three patients’ cortical windows were healed more than a half
(grade III) (Figure 3). The median MSTS score was 84.5 (range, 66 to 97). Nine
patients (28%) underwent prophylactic stabilization.
There
was no statistically significant difference between tumor size and prophylactic
fixation.
p=0.592).
However, there was a statistically significant difference between prophylactic
fixation and cortical window (p=0.013). There was no significant difference
between the usage of prophylactic fixation and new bone formation on the
cortical window (p=0.967). Postoperative first year MSTS score was found
statistically worse in patients with prophylactic fixation (p<0.001)
There
was a weak negative correlation between age and new bone formation (p=0.046, r=
-0.356). There was a moderate negative correlation between the return time to
work and MSTS score (p=0.004, r= -0.498). There was a moderate negative
correlation between cortical window and MSTS score (p=0.001, r= -0.577). There
was no correlation between age and MSTS score (p=0.223), tumor volume and MSTS
score (p=0.771), new bone formation and cortical window size (p=0.692), new
bone formation and MSTS score (p=0.964), the return time to work and new bone
formation on cortical window. (p=0.398)
Local
recurrence happened only in one patient, who had giant cell bone tumor. After
aggressive curettage, revision surgery was performed by applying DBM over the
cement, as the same procedure. None of the patients had any other complications
such as infection, pathological fracture, seroma or hematoma.
DISCUSSION
The
usage of cement in bone tumors provides immediate structural support to bone
and absorbing stress. Thermal cytotoxic effect of the cement reduces the local
recurrence. Cement reconstruction is a successful method in bone tumors because
it provides mechanical support and reduces the possibility of pathological
fracture. Therefore, it is more preferable than graft in the load bearing, high
stress regions, and large defects.Its simple reconstruction procedure, satisfactory
functional and radiological results as well as low-cost increase the range of
usage.
Demineralized
bone matrix is an alternative allograft product for filling bone defects, which
provides bone regeneration mainly through osteoconduction and partly osteoinduction. In
recent years, the treatment of benign bone tumors with DBM has become popular
in orthopedic and maxillofacial surgery due to its high recovery and low
complication rates.[10,11] Therefore, it has increased its combined use of
other graft materials. There are two level 3 and three level 4 studies, which
show that combined DBM and autologous bone marrow use is effective in the
treatment of active bone cysts. Successful results have been also shown with
the use of DBM in combination with steroids. Teng et al.[18] reported that
the combined use of allograft and cement in giant-cell bone tumors around the
knee has led to less mechanical failure and they suggest this method as an
optimal reconstruction strategy. In the literature review, we could not find
any data about the combined use of DBM and cement as well as the effect of new
bone formation in cortical window on functional scores.[19] By the cement
combined DBM treatment, we provide initial mechanical strength by taking
advantage of the load-bearing effect of the cement which makes early weight
bearing possible. Moreover, we optimize the cost effectivity and reduce the
possibility of graft resorption and fracture by using less DBM. In addition, we
also increase cortical bone formation that carries the load on removed cortical
window. In our study, we found that 3/32 patients had more than 50% new bone
formation on the cortical window and the cortical window of 10/32 patients were
almost completely healed with new bone in the first year CT scans. Although the
efficacy of DBM to produce live bone is best demonstrated only by histological
examination, the thin rim layer, which appears in tomography and direct graphs,
shows an increased activity in the scintigraphy. The radiological and histological
results are parallel to each other in experimental studies.[20] We did not find
any correlation between the functional scores and new bone formation.
It
is known that the effectivity of different brands of DBMs varies from each
other. The reasons of different results have been shown such as the
sterilization process, washing procedure, varying from donor to donor resulting
in differences between products, inherent BMP types, and different amounts of
graft. The fact that the US Food and Drug Administration (FDA) is not
performing standard controls for DBMs was shown to be not a surprise in the
diversity of DBM results. We think that we have standardized and optimized our
treatment, because we used same brand, which has shown superiority, and same
form DBM.
Traditionally,
aging decreases mesenchymal cell differentiation, collagen activity, bone
metabolism, and recipient aging declines the effect of allografts.[23] Also,
there is an increased risk of non-union with elder population in DBM-treated
patients with lumbar fusion.[24] We found a negative correlation between aging
and new bone formation on cortical window consistent with the literature.
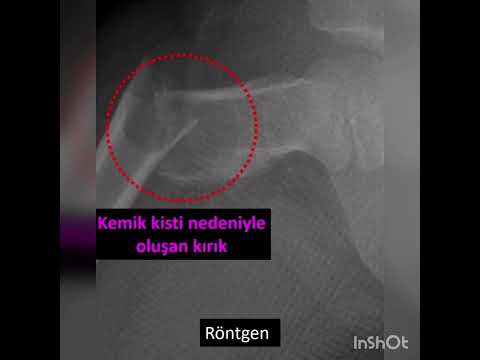
Prophylactic
osteosynthesis is indicated to reduce the possibility of pathological fracture
for bone tumors greater than 60 cm3 and in the load bearing areas.[25,26] We
did not find any correlation between.
tumor
volume and prophylactic fixation. However, no pathological fracture was seen in
any patient. In addition, we found a correlation between cortical window size and
the use of prophylactic fixation. The literature is unclear and open to
research about the relationship between the size of cortical window and the
need for prophylactic fixation. We believe that the complication of
pathological fracture can be prevented by this surgical technique using the
mechanical effect of cement and the biological effect of DBM. We found that
functional results were worse in patients undergoing prophylactic fixation.
However, it should not be disregarded that the functional outcomes are
relatively worse in a possible pathological fracture.
This
study has some limitations. First, removal of different types of tumors would
affect recurrence, time to return to work, and new bone formation on the
cortical window. Second, the distribution of the load on the lower and upper
extremities could not be the same; therefore, this may have affected the return
to work, new bone formation on the cortical window, and functional scores.
In
conclusion, the cement combined DBM treatment is a cost-effective, alternative
method in tumor surgery, that provides immediate stability and stimulates new
bone formation on cortical window. Although new bone formation is achieved on
cortical window with this method, new bone formation has not been found to
create a change in functional results. The transformation of the new bone to
the cortical bone and how long it lasts are open to research. We believe that
the histological evaluation of this method supported by controlled studies will
guide future tumor treatment methods.
Declaration of
conflicting interests
The
authors declared no conflicts of interest with respect to the authorship and/or
publication of this article.
Funding
The
authors received no financial support for the research and/or authorship of
this article.
REFERENCES
1.
Nogueira Drumond JM. Benign bone tumors and tumor[1]like bone lesions:
Treatment update and new trends. Rev Bras Ortop 2015;44:386-90.
2.
Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern
orthopaedic surgery. J Bone Joint Surg [Br] 2007;89:851-7.
3.
Hass HJ, Krause H, Kroker S, Wagemann W. Implantation of human demineralized
bone matrix (DBM) for the treatment of juvenile bone cysts. Oper Orthop
Traumatol 2006;18:19-33.
4.
Somville J, De Beuckeleer L, De Schepper A, Verstreken J, Taminiau A. Reliability
of measuring volume by different methods for tumors of the musculoskeletal
system. Acta Orthop Belg 2001;67:338-43.
5.
Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the
functional evaluation of reconstructive procedures after surgical treatment of
tumors of the musculoskeletal system. Clin Orthop Relat Res 1993;286:241-6.
6.
Martinez, M, Hwang, J, Beebe KS. Local adjuvants for benign aggressive bone
tumors, Current Orthopaedic Practice 2014;25:573-9.
7.
Szalay K, Antal I, Kiss J, Szendroi M. Comparison of the degenerative changes
in weight-bearing joints following cementing or grafting techniques in giant
cell tumour patients: medium-term results. Int Orthop 2006;30:505-9.
8.
Özer D, Er T, Aycan OE, Öke R, Coşkun M, Kabukçuoğlu YS. May bone cement be
used to treat benign aggressive bone tumors of the feet with confidence? Foot
(Edinb) 2014;24:1-5.
9.
Urist MR, Strates BS. Bone formation in implants of partially and wholly
demineralized bone matrix. Including observations on acetone-fixed intra and
extracellular proteins. Clin Orthop Relat Res 1970;71:271-8.
10.
Drosos GI, Touzopoulos P, Ververidis A, Tilkeridis K, Kazakos K. Use of
demineralized bone matrix in the extremities. World J Orthop 2015;6:269-77.
11.
Dinopoulos H, Dimitriou R, Giannoudis PV. Bone graft substitutes: What are the
options? Surgeon 2012;10:230-9.
12.
Di Bella C, Dozza B, Frisoni T, Cevolani L, Donati D. Injection of
demineralized bone matrix with bone marrow concentrate improves healing in
unicameral bone cyst. Clin Orthop Relat Res 2010;468:3047-55.
13.
Park IH, Micic ID, Jeon IH. A study of 23 unicameral bone cysts of the
calcaneus: open chip allogeneic bone graft versus percutaneous injection of
bone powder with autogenous bone marrow. Foot Ankle Int 2008;29:164-70.
14.
Kanellopoulos AD, Yiannakopoulos CK, Soucacos PN. Percutaneous reaming of
simple bone cysts in children followed by injection of demineralized bone
matrix and autologous bone marrow. J Pediatr Orthop 2005;25:671-5
15.
Docquier PL, Delloye C. Treatment of aneurysmal bone cysts by introduction of
demineralized bone and autogenous bone marrow. J Bone Joint Surg [Am]
2005;87:2253-8.
16.
Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with
percutaneous injection of demineralized bone matrix and autogenous bone marrow.
J Bone Joint Surg [Am] 2002;84:921-9.
17.
Sung AD, Anderson ME, Zurakowski D, Hornicek FJ, Gebhardt MC. Unicameral bone
cyst: a retrospective study of three surgical treatments. Clin Orthop Relat Res
2008;466:2519-26.
18.
Teng W, Lin P, Li Y, Yan X, Li H, Li B, et al. Bone combined cement grafting in
giant cell tumor around the knee reduces mechanical failure. Int Orthop
2019;43:475-82.
19.
Atik OŞ. Which articles do we prefer to publish?. Eklem Hastalik Cerrahisi 2018;29:1.
20.
Enneking WF, Campanacci DA. Retrieved human allografts: a clinicopathological
study. J Bone Joint Surg [Am] 2001;83:971-86.
21.
Peterson B, Whang PG, Iglesias R, Wang JC, Lieberman JR. Osteoinductivity of
commercially available demineralized bone matrix. Preparations in a spine
fusion model. J Bone Joint Surg [Am] 2004;86:2243-50.
22.
Sammarco VJ, Chang L. Modern issues in bone graft substitutes and advances in
bone tissue technology. Foot Ankle Clin 2002;7:19-41.
23.
Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of
human bone marrow cells cultured in threedimensional collagen sponges. J Cell
Biochem 2001;82:583-90.
24.
Ajiboye RM, Eckardt MA, Hamamoto JT, Sharma A, Khan AZ, Wang JC. Does Age
Influence the Efficacy of Demineralized Bone Matrix Enriched with Concentrated
Bone Marrow Aspirate in Lumbar Fusions?. Clin Spine Surg 2018;31:E30-E5.
25.
Perisano C, Barone C, Stomeo D, Di Giacomo G, Vasso M, Schiavone Panni A, et
al. Indications for prophylactic osteosynthesis associated with curettage in
benign and low-grade malignant primitive bone tumors of the distal femur in
adult patients: a case series. J Orthop Traumatol 2016;17:377-82.
26.
Kornah B, Safwat H, Ghany TA, Aal MA, Saleem N. Prophylactic Fixation of
Impending Fractures. MOJ Orthop Rheumatol 2016;6:00206
 Sprache
Sprache Türkçe
Türkçe English
English Arabic
Arabic Germany
Germany Russian
Russian